Publications
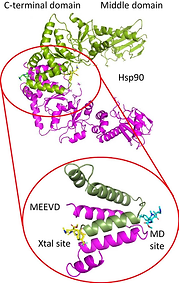
31. Jeanne, X., Oberoi, J., Roe, M. S., Baud, M., Spencer, J., Torok, Z., Vigh, L., Prodromou, C.
The dihydropyridine LA1011 modulates multiple Hsp90 co- chaperone interactions relevant to Alzheimer’s disease
Cell Stress and Chaperones, 31, 1, 100131(2025)
DOI: 10.1016/j.cstres.2025.100131

30. Khadiullina, R., Chasov, V., Gilyazova, E., Davletshin, D., Mirgayazova, R., Mingaleeva, R.,Stephenson Clarke, J.
R., Baud, M. G. J., Bulatov, E.
Cellular activity upregulation of the thermolabile p53
cancer mutant Y220C by small molecule indazole
derivatives
Cell Death Discovery, 11, 508 (2025)
DOI: 10.1038/s41420-025-02781-6

29. Hayward, L., Baud, M. G. J.
Cysteine sulfinic acid and sulfinylated peptides
RSC Chemical Biology, 6, 1019–1033 (2025)
DOI: 10.1039/D5CB00040H

28. Moraru, R., Valle-Argos, B., Minton, A., Buermann, L., Pan,
S., Wales, T. M., Joseph, R. E., Andreotti, A., Strefford, J. C.,
Packham, G., Baud, M. G. J.
Exploring 2-Sulfonylpyrimidine Warheads as Acrylamide
Surrogates for Targeted Covalent Inhibition: A BTK Story
Journal of Medicinal Chemistry, 67, 13572–13593 (2024)
DOI: 10.1021/acs.jmedchem.3c01927

27. Pichon, M., Drelinkiewicz, D., Lozano, D., Moraru, R., Hayward,
L., Jones, M., McCoy, M., Allstrum-Graves, S., Balourdas, D-I.,
Joerger, A., Whitby, R., Goldup, S., Wells, N., Langley, G.,
Herniman, J., Baud, M. G. J.
Structure–Reactivity Studies of 2-Sulfonylpyrimidines Allow
Selective Protein Arylation
ACS Bioconjugate Chemistry, 34, 9, 1679–1687 (2023)
DOI: 10.1021/acs.bioconjchem.3c00322
ChemRxiv. Cambridge: Cambridge Open Engage (2023)
DOI: 10.26434/chemrxiv-2023-cx8vk

26. Schlotawa, L., Tyka, K., Kettwig, M., Ahrens‐Nicklas, R.C., Baud,
M., Berulava, T., Brunetti‐Pierri, N., Gagne, A., Herbst, Z.M.,
Maguire, J.A. and Monfregula, J.,
Drug screening identifies tazarotene and bexarotene as
therapeutic agents in multiple sulfatase deficiency
EMBO Molecular Medicine,e14837, (2023)
DOI: 10.15252/emmm.202114837

25. Stephenson Clarke, J. R., Douglas, L. R., Duriez, P. J.,
Balourdas D-I., Joerger, A. C., Khadiullina, R., Bulatov, E.,
Baud, M. G. J.
Discovery of Nanomolar-Affinity Pharmacological Chaperones
Stabilizing the Oncogenic p53 Mutant Y220C
ACS Pharmacology and Translational Science,
5, 1169-1180 (2022)
DOI: 10.1021/acsptsci.2c00164

24. McCoy, M. A., Spicer, D., Wells, N., Hoogewijs, K., Fiedler,
M., Baud, M. G. J.
Biophysical Survey of Small-Molecule β-Catenin Inhibitors: A Cautionary Tale
Journal of Medicinal Chemistry, 65, 7246-7261 (2022)
DOI: 10.1021/acs.jmedchem.2c00228


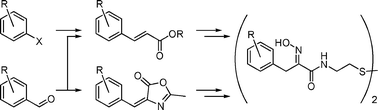
22. Troup, R. I., Fallan, C., Baud, M. G. J.
Current strategies for the design of PROTAC linkers: a critical review
Exploration of Targeted Anti-tumor Therapy, 1, 273-312, (2020)
DOI: 10.37349/etat.2020.00018

21. Chasov, V., Mirgayazova, R., Zmievskaya, E., Khadiullina, R., Valiullina,
A., Stephenson Clarke, J., Rizvanov, A., Baud, M., Bulatov, E.
Key Players in the Mutant p53 Team: Small Molecules, Gene
Editing, Immunotherapy
Frontiers in Oncology, 10, 1460, (2020).
DOI: 10.3389/fonc.2020.01460

20. Schlotawa, L., Kettwig, M., Radhakrishnan, K., Dierks, T.,
Gartner, J. and Baud, M.,
Method for the treatment of diseases associated with sulfatase
deficiencies.
(2020), US application pending.
US20210100756A1
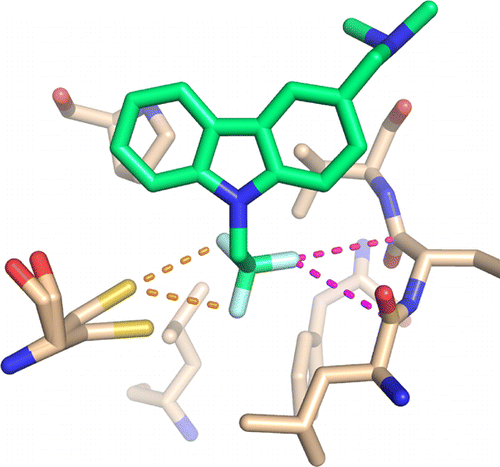
19. Baud, M. G. J., Bauer, M. R., Verduci, L., Dingler, F. A., Patel,
K. J., Horil Roy, D., ... Fersht, A. R.
Aminobenzothiazole derivatives stabilize the thermolabile p53 cancer mutant Y220C and show anticancer activity in p53- Y220C cell lines.
European Journal of Medicinal Chemistry, 152, 101-114, (2018)
DOI: 10.1016/j.ejmech.2018.04.035
18. Runcie, A. C., Zengerle, M., Chan, K-H., Testa, A., van
Beurden, L., Baud, M. G. J., ... Ciulli, A.
Optimization of a “bump-and-hole” approach to allele-selective
BET bromodomain inhibition.
Chemical Science, (2018)
DOI: 10.1039/C7SC02536J
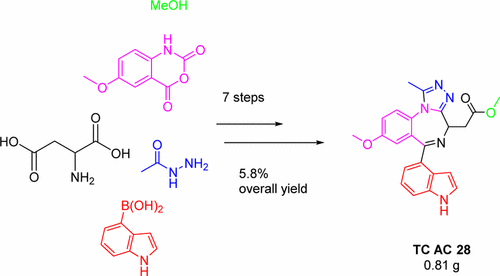
17. Khan, R., Marsh, G., Felix, R., Kemmitt, P. D., Baud, M. G. J.,
Ciulli, A., & Spencer, J.
Gram scale laboratory synthesis of TC AC 28, a high affinity
BET bromodomain ligand.
ACS Omega, 2(8), 4328-4332, (2017)
DOI: 10.1021/acsomega.7b00780
16. Weston, C. E., Krämer, A., Colin, F., Yildiz, Ö., Baud, M. G. J.,
Meyer-Almes, F-J., & Fuchter, M. J.
Toward photopharmacological antimicrobial chemotherapy
using photoswitchable amidohydrolase inhibitors.
ACS Infectious Diseases, 3(2), 152-161, (2017)
DOI: 10.1021/acsinfecdis.6b00148
15. Bauer, M. R., Jones, R. N., Baud, M., Wilcken, R., Boeckler, F.
M., Fersht, A. R., ... Spencer, J.
Harnessing fluorine–sulfur contacts and multipolar interactions
for the design of p53 mutant Y220C rescue drugs.
ACS Chemical Biology, 11(8), 2265-2274, (2016)
DOI: 10.1021/acschembio.6b00315
14. Baud, M., Lin-Shiao, E., Zengerle, M., Tallant, C., & Ciulli, A.
New synthetic routes to triazolo-benzodiazepine analogues: expanding the scope of the bump-and-hole approach for
selective bromo and extra-terminal (bet) bromodomain inhibition.
Journal of Medicinal Chemistry, 59(4), 1492-1500, (2016)
DOI: 10.1021/acs.jmedchem.5b01135
13. Shiao, E. L., Baud, M. G. J., Cuilli, A., Chan, K-H., Zengerle, M.,
Enzyme Functional Probes
(2015)
WO 2015/079259
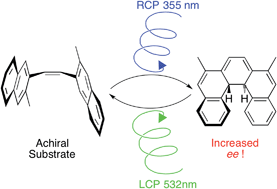
12. Richardson, R. D., Baud, M., Weston, C. E., Rzepa, H. S.,
Kuimova, M. K., & Fuchter, M. J.
Dual wavelength asymmetric photochemical synthesis with
circularly polarized light.
Chemical Science, 6(7), 3853-3862, (2015)
DOI: 10.1039/C4SC03897E
11. Joerger, A. C., Bauer, M. R., Wilcken, R., Baud, M., Harbrecht,
H., Exner, T. E., ... Fersht, A. R.
Exploiting transient protein states for the design of small-molecule stabilizers of mutant p53.
Structure, 23(12), 2246-2255, (2015)
10. Baud, M. G. J., Lin-Shiao, E., Cardote, T., Tallant, C.,
Pschibul, A., Chan, K-H., ... Ciulli, A.
A bump-and-hole approach to engineer controlled selectivity of BET bromodomain chemical probes.
Science, 346(6209), 638-641, (2014)
9. Meyners, C., Baud, M., Fuchter, M. J., & Meyer-Almes, F-J.
Analytical Biochemistry, 460, 39-46, (2014)
8. Dias, D. M., Van Molle, I., Baud, M., Galdeano, C.,
Geraldes, C. F. G. C., & Ciulli, A. Letter.
Is NMR fragment screening fine-tuned to assess druggability of protein–protein interactions?
ACS Medicinal Chemistry Letters, 5(1), 23-28, (2014)
DOI: 10.1021/ml400296c
7. Meyners, C., Baud, M., Fuchter, M. J., & Meyer-Almes, F-J.
Thermodynamics of ligand binding to histone deacetylase like amidohydrolase from Bordetella/Alcaligenes.
Journal of Molecular Recognition, 27(3), 160-172, (2014)
DOI: 10.1002/jmr.2345
6. Baud, M., Leiser, T., Petrucci, V., Gunaratnam, M.,
Neidle, S., Meyer-Almes, F-J., & Fuchter, M. J.
Thioester derivatives of the natural product psammaplin A as potent histone deacetylase inhibitors.
Beilstein Journal of Organic Chemistry, 9, 81-88, (2013)
DOI: 10.3762/bjoc.9.11
5. Baud, M., Leiser, T., Haus, P., Samlal, S., Wong, A. C.,
Wood, R. J., Fuchter, M. J.
Defining the mechanism of action and enzymatic selectivity of psammaplin A against its epigenetic targets.
Journal of Medicinal Chemistry, 55(4), 1731-1750, (2012)
DOI: 10.1021/jm2016182
4. Baud, M., Haus, P., Leiser, T., Meyer-Almes, F-J., &
Fuchter, M. J.
Highly ligand efficient and selective N-2-(thioethyl)picolinamide histone deacetylase inhibitors inspired by the natural product psammaplin A.
ChemMedChem, 8(1), 149-156, (2012)
3. Baud, M., Leiser, T., Meyer-Almes, F-J., & Fuchter, M. J.
New synthetic strategies towards psammaplin A, access
to natural product analogues for biological evaluation.
Organic & Biomolecular Chemistry, 9(3), 659-662, (2011)
DOI: 10.1039/C0OB00824A
2. Borcard, F., Baud, M., Bello, C., Dal Bello, G., Grossi, F.,
Pronzato, P., ... Vogel, P.
Synthesis of new oxathiazinane dioxides and their in vitro cancer cell growth inhibitory activity.
Bioorganic & Medicinal Chemistry Letters, 20(17), 5353-5356, (2010)
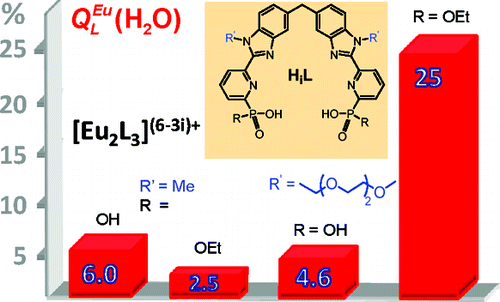
1. Chauvin, A-S., Comby, S., Baud, M., De Piano, C., Duhot,
C., & Bünzli, J-C. G.
Luminescent lanthanide helicates self-assembled from ditopic ligands bearing phosphonic acid or phosphoester units.
Inorganic Chemistry, 48(22), 10687-10696, (2009)
DOI: 10.1021/ic901424w










.png)


.jpg)